Professor John Isaacs, the trust’s Director of Research and Associate Medical Director, celebrates the registration of our 10,000th study
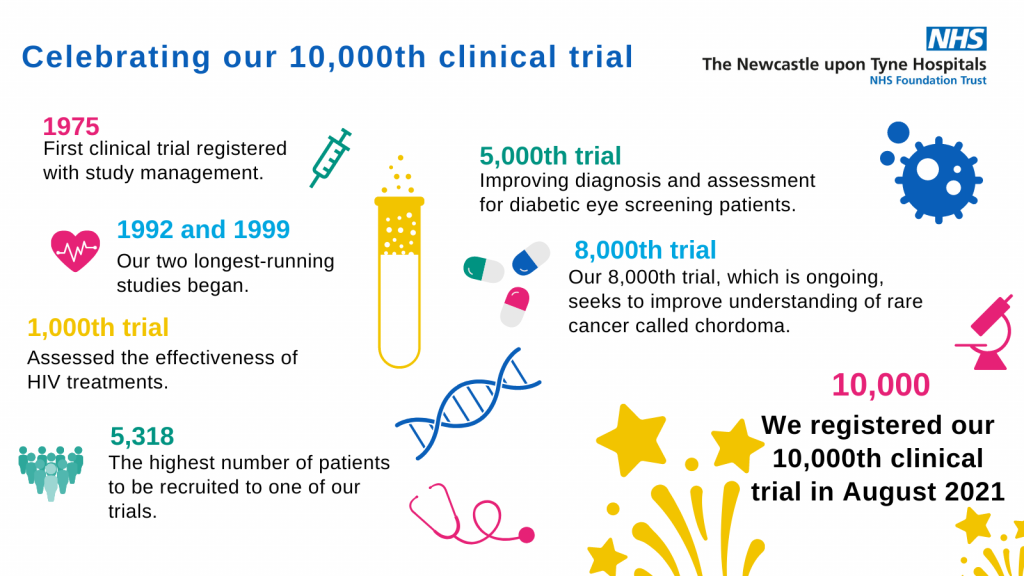
In 1975, our study management team registered one of the first clinical trials at Newcastle Hospitals. The study looked at how proteins on the surface of breast cancer cells contribute to the various different types of breast cancer and its potential to spread.
Fast forward 46 years later and we’ve just registered our 10,000th study, an incredible success that we want to celebrate.
The 10,000th study will test the effectiveness of a treatment called extracorporeal photopheresis (ECP) on patients who develop severe organ rejection following a lung transplant.
ECP is a type of therapy which is designed to stop a group of white blood cells, known as lymphocytes, attacking the body following an organ transplant. Sadly, some patients become very poorly with organ rejection after their lung transplant and don’t survive, however it’s hoped that this study could help to change this.
The study (NIHR 130612) is being led by Professor Andrew Fisher from the Institute of Transplantation at the Freeman Hospital and Newcastle University on behalf of all five lung transplant centres in the UK and is funded by the Efficacy and Mechanism Evaluation (EME) Programme, an MRC and NIHR partnership.
Today we recruit more than 10,000 patients into hundreds of studies each year. Some of these trials have led to scientific breakthroughs and the discovery of new medicines and treatment. You can take a look at just some of these below:
Of course, none of this would be possible without the passion and dedication of our teams who work tirelessly to run and manage clinical trials. We couldn’t do it without the efforts of everyone involved.
Take a look at what’s next for the future of research at Newcastle Hospitals by reading our clinical research strategy (2021-2026).
